RegenPRP™ - HA
Class III CE certified Medical Devices
under Regulation (EU) 2017/745.
Regen Lab SA is an ISO 13485:2016
and MDSAP certified medical device manufacturer

RegenPRP™ in combination with HA
1.1 – Platelet Rich Plasma
RegenPRP™: the platelet concentrate prepared with this Regenlab® technology provides an autologous reservoir of growth factors.
- Proven role in the healing of tissues, with key roles in cell migration, proliferation and differentiation
- Mechanism of action comprises anti-inflammatory activity and activation of cell-signalling cascades
- Key role in the synthesis of new extracellular matrix for tissue regeneration
1.2 – Hyaluronic Acid
Hyaluronic acid is a major component of synovial fluid contributing to joint homeostasis.
- 25 years of clinical experience shows pain relief and functional improvement lasting 6 to 12 months in OA patients.
- Plays a major role in viscosupplementation and pain relief in OA.4
- The network of HA chains generates an ideal cell-friendly matrix when combined with PRP.5
Breaking the vicious cycle of knee osteoarthritis
SYNERGISTIC ACTION

Pain Relief
Inflammation Reduction
De Novo HA Synthesis
Cell Moduclation
SYNEGISTIC ACTION
Pain Relief
Inflammation Reduction
Cell Moduclation
De Novo HA Synthesis
HA
PRP

Hyaluronic acid improves the activity of several molecules contained in platelet-rich plasma to provide additional bene ts to OA patients.1
Both PRP and HA have been extensively used not only to reduce clinical symptoms, but also to slow down OA progression.5,7
When PRP and HA are used in combination these effects are enhanced and prolonged. HA creates a bioactive sca old in which the platelets progressively release their growth factors. RegenPRP™ does not negatively affect the mechanical, elastic or viscous properties of HA.1
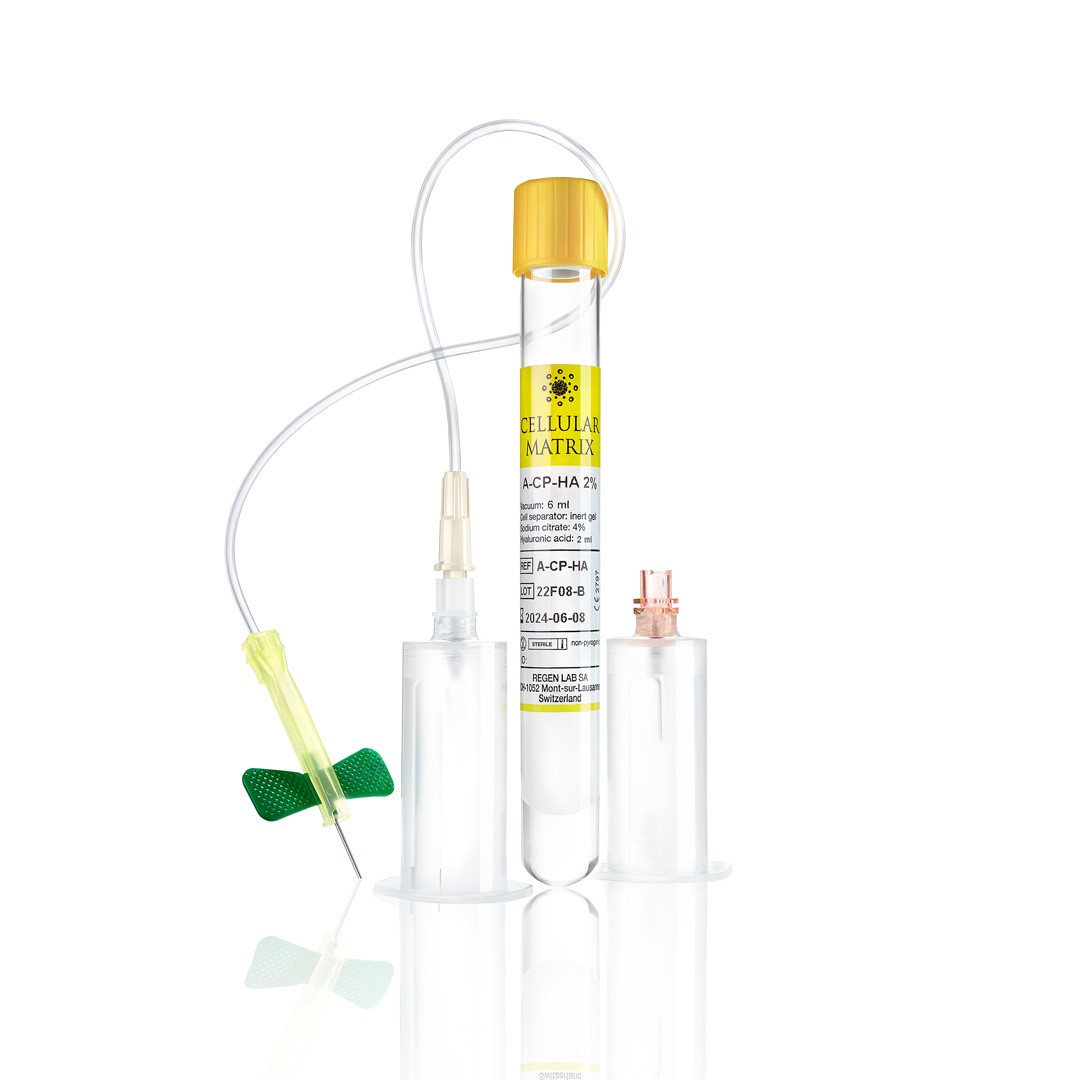
Cellular Matrix is a unique, regulatory approved, patented technology that allows the safe, closed-circuit preparation of a cell-friendly PRP-HA network in which platelets and plasma components are retained.
%
Platelet recovery
%
Granulocyte depletion
%
Red blood cell depletion
Hyaluronic acid improves the activity of several molecules contained in platelet-rich plasma to provide additional benefits to OA patients.*
Bibliography
1. Abate M. et al. E cacy and safety pro le of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur J Orthop Surg Traumatol 2015 Dec;25(8):1321-6.
2. Fountain, J. H. and S. L. Lappin (2019). Physiology, Platelet, StatPearls Publishing, Treasure Island (FL).
3. Mathew, J. and M. Varacallo (2019). Physiology, Blood Plasma, StatPearls Publishing, Treasure Island (FL).
4. Oeding, J. F., N. H. Varady, F. W. Fearington, A. Pareek, S. M. Strickland, B. U. Nwachukwu, C. L. Camp and A. J. Krych (2024). «Platelet-Rich Plasma Versus Alternative Injections for Osteoarthritis of the Knee: A Systematic Review and Statistical Fragility Index-Based Meta-analysis of Randomized Controlled Trials.» Am J Sports Med: 3635465231224463
5. Huskisson, E. C., and S. Donnelly. «Hyaluronic Acid in the Treatment of Osteoarthritis of the Knee.» [In eng]. Rheumatology (Oxford) 38, no. 7 (Jul 1999): 602-7.
6. Smith J.D. et al. Improved growth factor directed vascularisation into fibrin constructs through inclusion of additional extracellular molecules. Microvasc Res. 2007;73(2):84-94.
7. Chen CPC, Cheng CH, Hsu CC, Lin HC, Tsai YR, Chen JL. The in uence of platelet rich plasma on synovial fluid volumes, protein concentrations, and severity of pain in patients with knee osteoarthritis. Experimental gerontology 2017;93:68-72.
8. Renevier, J. L., et al. «Cellular Matrix PRP-HA”: A new treatment option with platelet-rich plasma and hyaluronic acid for patients with osteoarthritis having had an unsatisfactory clinical response to hyaluronic acid alone: Results of a pilot, multicenter French study with long-term follow-up.» Int. J. Clin. Rheumatol. 2018;13(4):230-8
9. Barac, B., et al. (The new treatment approach in knee osteoarthritis: Effcacy of cellular matrix combination of platelet rich plasma with hyaluronic acid versus two different types of hyaluronic acid (HA). Int. J. Clin. Rheumatol. 2018;13(5):289-95
