Patents
Intellectual Property Rights (IPRs) as Core AssetsAs a leader in the PRP&HA regenerative field and as an innovation-driven company, Regen Lab SA has devoted important resources since 2004 to put on the market pioneering and diversified products constituting a complete set of tools/technologies for the medical community. Innovation represents one of the strongest pillars of the company with nearly 100 in-house scientists and a unique network of talented and renowned medical doctors contributing to Regen Lab’s Research & Development.
Over nearly 20 years, this innovation has been secured by building substantial patent & trademarks portfolios. Intellectual Property Rights (IPRs) therefore represent core assets of the company protecting its unique products and forging Regen Lab’s renowned brand names.
The patent portfolio can be divided into two core technologies:
1-PRP/BMC gel devices, methods and uses thereof, alone or in combination with cell extracts and associated key technologies like Autologous Thrombin Serum (ATS) and PRP Cell Culture.
Associated trademarks are REGENLAB®, REGENKIT®, REGENACR®, PRP®, A-PRP®, REGENCELL®, REGENPLASMA®, REGEN PRP®, REGEN®, REGEN EXTRACELL®, THT®, cellularmask®, CUTECELL®, REGENVET®, A-CPKIT® which are registered trademarks of REGEN LAB SA in Europe, United States and other countries.
2-Hyaluronic acid alone, cross-linked or not, or in combination with PRP/BMC leading to the unique “all in one” Cellular Matrix products combining in a synergetic manner the advantageous properties of PRP and HA, but also preparation in two separate devices.
Associated trademarks are REGENLAB®, REGENKIT®, CELLULAR MATRIX® fig., SKINVISC®, ARTHROVISC®, REGENMATRIX®, REGEN®, PRP® and A-PRP® which are registered trademarks of REGEN LAB in Europe, United States and other countries.
July 24, 2024
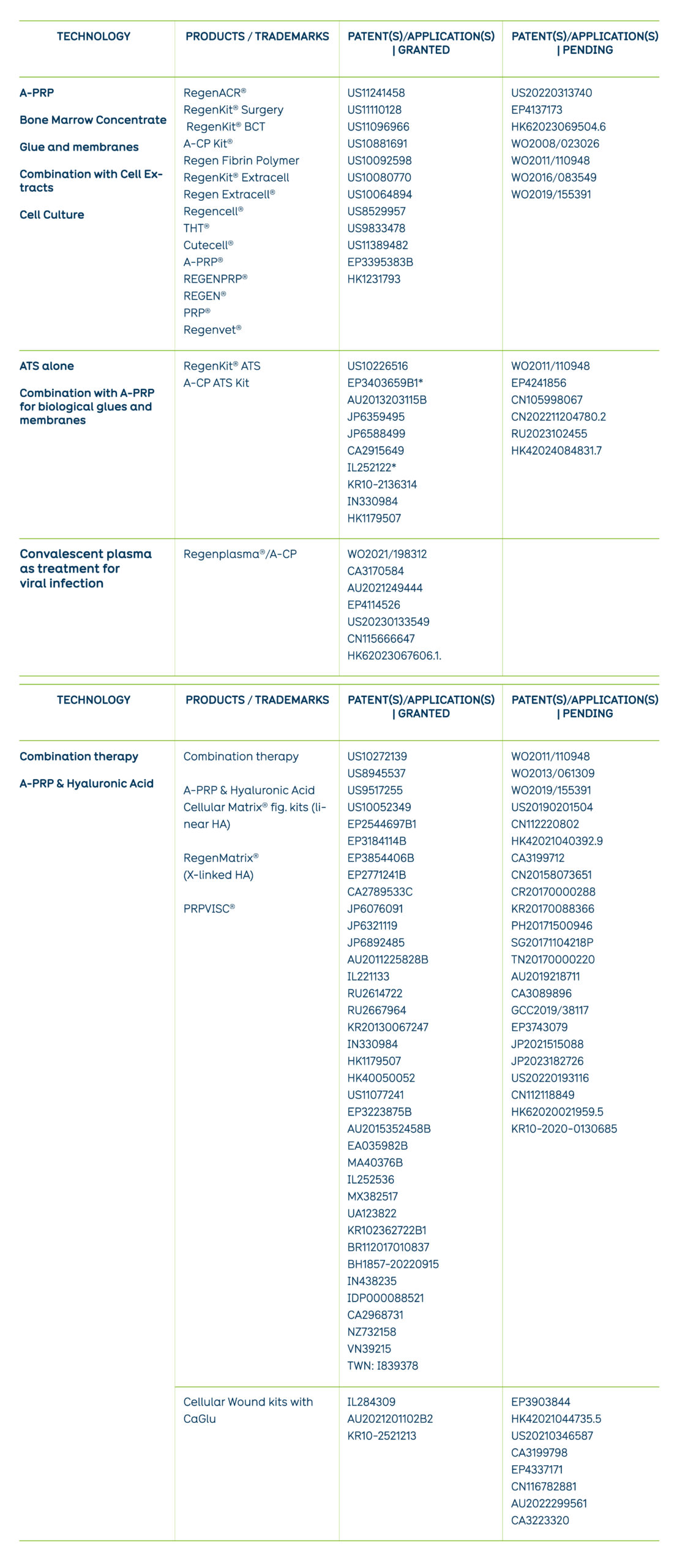
A-PRP
Bone Marrow Concentrate
Glue and membranes
Combination with Cell Extracts
Cell Culture
Products | Trademarks
RegenACR®
RegenKit® Surgery
RegenKit® BCT
A-CP Kit®
Regen Fibrin Polymer
RegenKit® Extracell
Regen Extracell®
Regencell®
THT®
Cutecell®
A-PRP®
REGENPRP®
REGEN®
PRP®
Regenvet®
Patent(s) | Granted
US11241458
US11110128
US11096966
US10881691
US10092598
US10080770
US10064894
US8529957
US9833478
US11389482
EP3395383B
HK1231793
Patent(s) | Pending
US20220313740
EP4137173
HK62023069504.6
WO2008/023026
WO2011/110948
WO2016/083549
WO2019/155391
ATS alone
Combination with A-PRP for biological glues and membranes
Products | Trademarks
RegenKit® ATS
A-CP ATS Kit
Patent(s) | Granted
US10226516
EP3403659B1*
AU2013203115B
JP6359495
JP6588499
CA2915649
IL252122*
KR10-2136314
IN330984
HK1179507
Patent(s) | Pending
WO2011/110948
EP4241856
CN105998067
CN202211204780.2
RU2023102455
HK42024084831.7
Combination therapy
A-PRP & Hyaluronic Acid
Products | Trademarks
Cellular Matrix® fig. kits (linear HA)
RegenMatrix® (X-linked HA)
PRPVISC®
Patent(s) | Granted
US10272139
US8945537
US9517255
US10052349
EP2544697B1
EP3184114B
EP3854406B
EP2771241B
CA2789533C
JP6076091
JP6321119
JP6892485
AU2011225828B
IL221133
RU2614722
RU2667964
KR20130067247
IN330984
HK1179507
HK40050052
US11077241
EP3223875B
AU2015352458B
EA035982B
MA40376B
IL252536
MX382517
UA123822
KR102362722B1
BR112017010837
BH1857-20220915
IN438235
IDP000088521
CA2968731
NZ732158
VN39215
TWN: I839378
Patent(s) | Pending
WO2011/110948
WO2013/061309
WO2019/155391
US20190201504
CN112220802
HK42021040392.9
CA3199712
CN20158073651
CR20170000288
KR20170088366
PH20171500946
SG20171104218P
TN20170000220
AU2019218711
CA3089896
GCC2019/38117
EP3743079
JP2021515088
JP2023182726
US20220193116
CN112118849
HK62020021959.5
KR10-2020-0130685
Combination therapy
A-PRP & Hyaluronic Acid
Products | Trademarks
Cellular Wound kits with CaGlu
Patent(s) | Granted
IL284309
AU2021201102B2
KR10-2521213
Patent(s) | Pending
EP3903844
HK42021044735.5
US20210346587
CA3199798
EP4337171
CN116782881
AU2022299561
CA3223320
Viral infections (Covid 19)
Products | Trademarks
Regenplasma®/A-CP
Patent(s) | Granted
WO2021/198312
CA3170584
AU2021249444
EP4114526
US20230133549
CN115666647
HK62023067606.1.
Patent(s) | Pending
EP3903844
HK42021044735.5
US20210346587
CA3199798
EP4337171
CN116782881
AU2022299561
CA3223320
July 17, 2024
